

The innovative photocatalyst, Rh/InGaN1-xOx, which uses solar energy to convert greenhouse gases into valuable chemicals, represents a major leap in sustainable chemical production. This nanostructure consists of rhodium nanoparticles on indium gallium nitride nanowires, enhancing the efficiency of reforming dry methane using carbon dioxide. Credit: SciTechDaily.com
A new photocatalyst developed by Shanghai Jiao Tong University offers a green and efficient way to convert greenhouse gases into chemicals using solar energy, representing a major advance in sustainable chemical production.
The new photocatalyst, called Rh/InGaN1-SHeys, is a nanostructure consisting of rhodium nanoparticles immobilized on oxygen-modified indium gallium nitride nanowires grown on silicon substrates. Under concentrated solar illumination, this composite material shows outstanding performance for dry reformation of methane (DRM) with carbon dioxide2achieving a synthetic gas evolution rate of 180.9 mmol gcat-1 H-1 With a selectivity of 96.3%. This represents a significant improvement over conventional catalytic systems, which often require high energy input and suffer from rapid deactivation.
“Our work represents a major step forward in addressing the dual challenges of greenhouse gas emissions and sustainable energy production,” said Professor Baoyin Zhou, lead researcher from Shanghai Jiao Tong University. “By harnessing the power of solar energy and rationally designed nanoengineering, we have demonstrated a green and efficient route to convert waste gases into valuable chemical resources.”
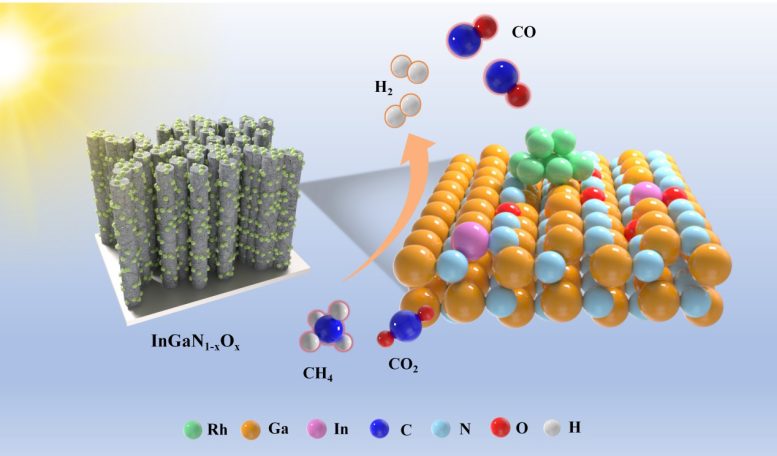
t/engan1-SHeys Nanowires have been explored for light-driven reformation of dry methane with carbon dioxide toward syngas (CH4 + company2 + Light = 2CO + 2H2). It is suggested that partial replacement of N in InGaN with O can significantly improve the activity and stability of the catalyst under light illumination without additional heating. Credit: China Science Press
Synergistic effects and mechanistic insights
The researchers attribute the exceptional performance of their photocatalyst to synergistic effects arising from the combination of photoactive InGaN nanowires, an oxygen-modified surface, and catalytically active rhodium nanoparticles. Mechanistic studies revealed that incorporated oxygen atoms play a crucial role in CO2 enhancement2 Activation, facilitation of carbon dioxide generation, and suppression of catalyst deactivation by coke deposition.
The results of this research, published in the prestigious journal Science Bulletin, pave the way for the development of advanced photocatalytic systems for the sustainable production of fuels and chemicals from renewable resources. The team believes their approach can be extended to other important chemical reactions, providing new opportunities for greening the chemical industry.
“We are excited about the prospects of this technology,” said Professor Baoyin Zhou. “By further improving the catalyst design and reactor configuration, we aim to scale up the process and demonstrate its feasibility for practical applications.”
Reference: “Rh/InGaN1−xOx nanostructure for photo-driven methane reforming with CO2 toward syngas” by Yixin Li, Jinglin Li, Tianqi Yu, Liang Qiu, Syed M. Najeeb Hassan, Lin Yao, Hu Pan, Shams Al-Irfan, Sherif. Muhammad Sadaf, Li Zhou, and Baoyin Zhou, February 12, 2024, Science Bulletin.
doi: 10.1016/j.scib.2024.02.020

“Web maven. Infuriatingly humble beer geek. Bacon fanatic. Typical creator. Music expert.”





More Stories
SpaceX launches 23 Starlink satellites from Florida (video and photos)
A new 3D map reveals strange, glowing filaments surrounding the supernova
Astronomers are waiting for the zombie star to rise again