

Map of the crystal structure of the alloy made from electron backscatter diffraction in a scanning electron microscope. Each color represents a portion of the crystal where the repeating structure changes its 3D orientation. Credit: Berkeley Lab
Researchers have discovered an unusual mineral Alloy It will not crack at extreme temperatures due to bending or bending of the crystals in the alloy at the atomic level.
A metal alloy composed of niobium, tantalum, titanium and hafnium has shocked materials scientists with its amazing strength and toughness at extremely hot and cold temperatures, a combination of properties that until now seemed almost impossible to achieve. In this context, strength is defined as the amount of force a material can withstand before it is permanently deformed from its original shape, and toughness is its resistance to breaking (cracking). The alloy's resilience to bending and breaking across a huge range of conditions could open the door to a new class of materials for next-generation engines that can operate more efficiently.
The team, led by Robert Ritchie at Lawrence Berkeley National Laboratory (Berkeley Laboratory) and UC Berkeley, in collaboration with groups led by Professors Deran Apelian at UC Irvine and Enrique Lavernia at Texas A&M University, discovered and then discovered the amazing properties of the alloy. How do they arise from interactions in atomic structure? Their work was described in a study recently published in the journal Sciences.
“The efficiency of converting heat into electricity or propulsion is determined by the temperature at which the fuel is burned—the hotter, the better. However, the operating temperature is limited by the structural materials it must withstand.” student in Ritchie’s lab. We have exhausted the ability to improve the materials we currently use at high temperatures, and there is a great need for new metallic materials. This is what this alloy promises.”
The alloy in this study is from a new class of metals known as high or medium temperature resistant alloys (RHEAs/RMEAs). Most metals we see in commercial or industrial applications are alloys made of one parent metal mixed with small amounts of other elements, but RHEAs and RMEAs are made by mixing near-equal amounts of metallic elements with very high melting temperatures, giving them still unique properties. Scientists discover it. Ritchie's group has studied these alloys for several years because of their potential for high-temperature applications.
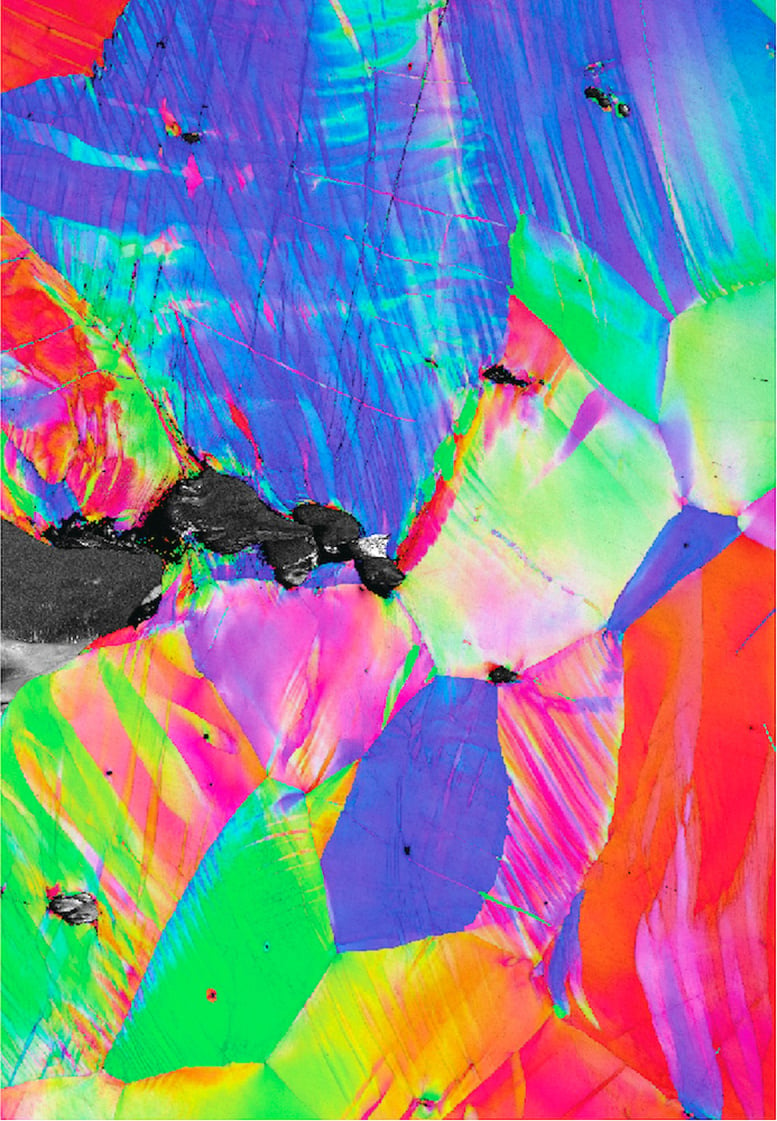
This material structure map shows lattice bands that form near the crack tip as cracks propagate (from left to right) in the alloy at 25°C, room temperature. Made using an electron backscatter diffraction detector in a scanning electron microscope. Credit: Berkeley Lab
“Our team has done previous work on RHEAs and RMEAs and found that these materials are very strong, but generally have very low fracture toughness, which is why we were shocked when this alloy showed exceptionally high toughness,” said the co-author. Puneet Kumar, a postdoctoral researcher in the group.
According to Cook, most RMEA have a fracture toughness of less than 10 MPa, making them some of the most brittle metals of all. The best cryogenic steels, specially designed to resist breakage, are about 20 times stronger than these materials. However, niobium, tantalum, titanium, and hafnium (Nb45Ta25T15High frequency15) The RMEA alloy was able to outperform even cryogenic steel, recording performance more than 25 times stronger than typical RMEA at room temperature.
But engines do not operate at room temperature. Scientists evaluated strength and durability at five total temperatures: -196°C (liquid nitrogen temperature), 25°C (room temperature), 800°C, 950°C, and 1200°C. The latter temperature is about 1/5 the temperature of the Sun's surface.
The team found that the alloy has its highest strength in the cold and becomes slightly weaker as temperature rises, but still has impressive numbers throughout the broad range. Fracture toughness, which is calculated from the amount of force needed to propagate an existing crack in a material, was high at all temperatures.
Revealing atomic arrangements
Almost all metal alloys are crystalline, meaning that the atoms within the material are arranged in repeating units. However, no crystal is perfect, they all contain imperfections. The most prominent defect that moves is called a dislocation, which is an imperfect plane of atoms in the crystal. When force is applied to metal, it causes several dislocations to move to accommodate the shape change.
For example, when you bend an aluminum paper clip, the movement of the dislocations within the paper clip accommodates the shape change. However, movement of dislocations becomes more difficult at low temperatures, and as a result many materials become brittle at low temperatures because the dislocations cannot move. That's why the Titanic's steel hull broke when it hit an iceberg. High-melting temperature elements and their alloys take this to the extreme, with many remaining brittle even at up to 800°C. However, this RMEA bucks the trend, withstanding interruption even at temperatures as low as liquid nitrogen (-196°C).
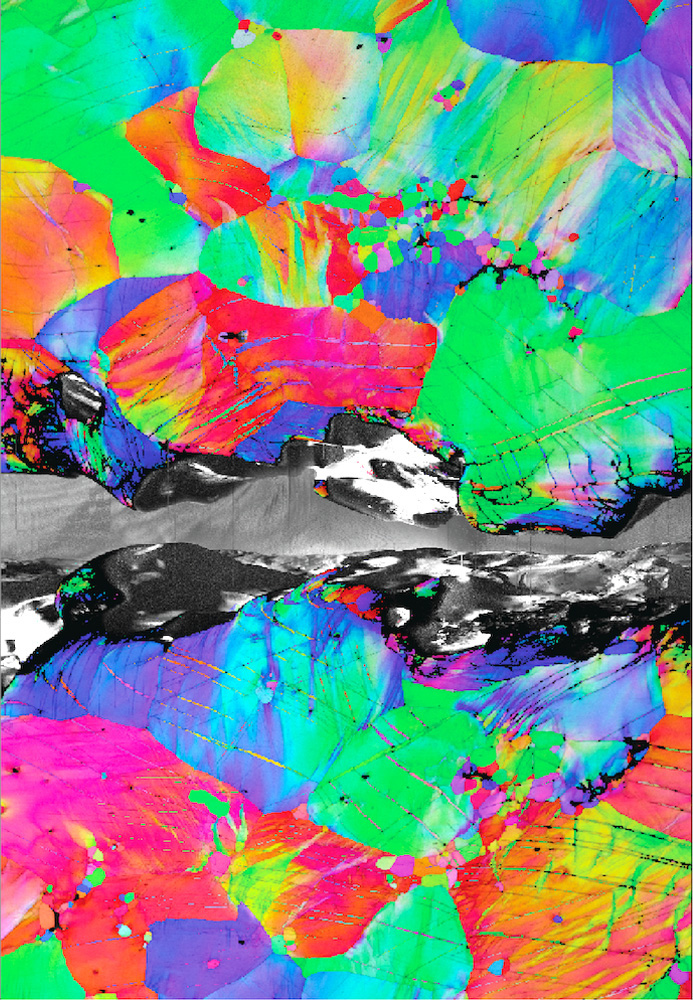
This map shows lattice bands formed near the crack tip during a crack propagation test (from left to right) in the alloy at -196°C. Credit: Berkeley Lab
To understand what was happening inside the exquisite metal, co-researcher Andrew Minor and his team analyzed the stressed samples, along with unbent and uncracked control samples, using a 4-dimensional scanning electron microscope (4D-STEM) and a scanning electron microscope (STEM) at the National Center for Electron Microscopy. , part of Berkeley Lab's Molecular Foundry.
Electron microscope data revealed that the alloy's unusual hardness comes from an unexpected side effect of a rare defect called a kink band. Knot bands are formed in a crystal when an applied force causes the segments of the crystal to suddenly collapse in on themselves and bend. The direction in which the crystal bends in these strands increases the force felt by the dislocations, making them move more easily. At the mass level, this phenomenon causes the material to soften (meaning that less force must be applied to the material as it deforms). The team knew from previous research that knot bands form easily in RMEA, but they hypothesized that the softening effect would make the material less rigid by making it easier for cracks to propagate through the network. But in reality, this is not the case.
“We have shown, for the first time, that in the case of a sharp crack between atoms, torsion bands actually resist crack propagation by distributing the damage away from it, preventing fracture and resulting in unusually high fracture toughness,” Cook said.
NB45Ta25T15High frequency15 The alloys will need to undergo more fundamental research and engineering testing before anything like a jet turbine or SpaceX The rocket nozzle is made of it, Ritchie said, because mechanical engineers really need to have a deep understanding of how their materials perform before using them in the real world. However, this study suggests that the metal has the potential to build the engines of the future.
Reference: “Kink bands enhance exceptional fracture resistance in refractory medium-entropy alloy NbTaTiHf” by David H. Cook, Punit Kumar, Madelyn I. Payne, Calvin H. Belcher, Pedro Borges, Wenqing Wang, Flynn Walsh, Zehao Li, Arun Devaraj , Mingwei Zhang, Mark Asta, Andrew M. Minor, Enrique J. Lavernia, Deran Abelian and Robert O. Richie, April 11, 2024, Sciences.
doi: 10.1126/science.adn2428
This research was conducted by David H. Cook, Puneet Kumar, and Madeleine I. Payne, and Calvin H. Belcher, Pedro Borges, Wenqing Wang, Flynn Walsh, Zihao Li, Arun Devaraj, Mingwei Zhang, Mark Asta, Andrew M. Minor, and Enrique. J. Lavernia, Deran Abelian, and Robert O. Ritchie, scientists at Berkeley Lab, UC Berkeley, Pacific Northwest National Laboratory, and UC Irvine, with funding from the Department of Energy's Office of Science. The experimental and computational analysis was performed at the Molecular Foundry and the National Energy Research Scientific Computing Center—both user facilities of the Department of Energy's Office of Science.

“Web maven. Infuriatingly humble beer geek. Bacon fanatic. Typical creator. Music expert.”





More Stories
SpaceX launches 23 Starlink satellites from Florida (video and photos)
A new 3D map reveals strange, glowing filaments surrounding the supernova
Astronomers are waiting for the zombie star to rise again